Thermal Radiation & Atomic Structure

| H. E. Smith | Winter 2007 |

| Physics 7 - Lecture Summary #5 Thermal Radiation & Atomic Structure |
 |

Thermal Radiation
Any object that is hot gives off light known as Thermal Radiation (or sometimes Blackbody Radiation for arcane physical reasons). The hotter an object is, the more light it emits. And, as the temperature of the object increase, it emits most of its light at higher and higher energies. (Higher energy light means shorter wavelength light.) The relationship between the amount of light emitted, its wavelength and its temperature is an equation known as the Planck Law,named after the German physicist Max Planck, who first discovered it. For a hot object at a given temperature, T, the equation gives the amount of light emitted at each wavelength. A plot of the Planck Law has a characteristic shape:
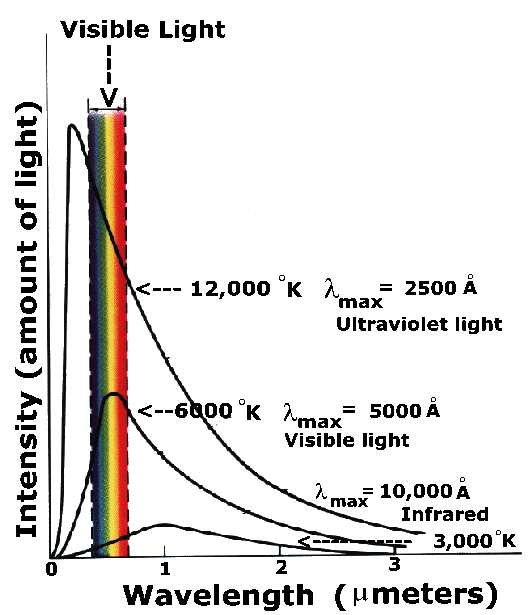
The peak wavelength of the curve is given by the formula:
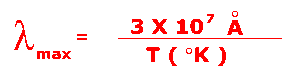
with wavelength in angstroms (Å) and temperature in degrees Kelvin. Here is a Java Thermal Radiation Experiment which will allow you to determine the relationship between temperature and wavelength. The table below shows some astronomical objects, their normal temperatures, and the wavelengths where they emit the most light.
| Thermal Radiation from Astronomical Objects | |||
|---|---|---|---|
| Object | Temperature (K) |
Peak Wavelength |
Region |
| Cosmic Background |
3 | 1mm | Microwave (IR-Radio) |
| Molecular Cloud |
10 | 300µm | Infrared |
| Humans | 310 | 9.7µm | Infrared |
| Incandescent Light Bulb |
3000 | 1µm 10,000Å |
IR/Visible |
| Sun | 6000 | 5000Å | Visible |
| Hot Star | 30,000 | 1000Å | Ultraviolet |
| Intra-Cluster Gas |
108 | 0.3Å | X-Ray |
If you add up the light energy emitted at all wavelengths, you get the total radiant energy from the object, called the Luminosity in Astronomy. The luminosity of an object depends very sensitively on temperature and also on size (surface area), so that:
 |
Physics at the microscopic (atomic) level is very different and seemingly very strange for those of us accustomed to the world on millimeter--kilometer scales. In our discussion, we'll employ a very simplified atomic model proposed in 1915 by Danish Nobel Laureate, Neils Bohr. This is called the "planetary atom" or Bohr Model. In this view, the atom consists of a nuclues, composed of protons and neutrons, with electrons orbiting around the nucleus, like a miniature solar-system. The electrical attraction between the protons and electrons holds the atom together. The typical size of an atomic nucleus is 10-13cm with the electrons orbiting at a radius of about 10-8cm (1Å). A realistic picture of atomic structure can be found at this UCLA site. |
When scientists began probing the structure of the atom in the late 1800's and early 1900's, they discovered an amazing thing. The standard rules of Physics, set forth by Isaac Newton, don't work at the atomic scale. Newtonian mechanics cannot correctly describe the behavior of protons, neutrons, electrons or atoms. Bohr, Planck, Pauli and other physicists (yes, including Einstein) began to develop a new set of physical "laws" that they demonstrated apply to the microscopic world of atoms. This new theory was called "Quantum Mechanics".
The Rules of Quantum Mechanics
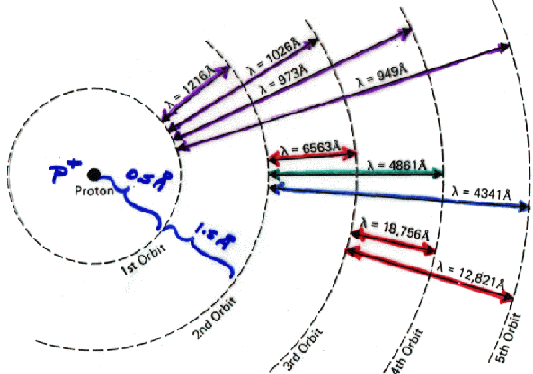
The energies available to an atom depend directly on the electrical force between the nucleus and the electrons. The difference in energy between levels corresponds to a specific wavelength of light. When the atom encounters a photon of this specific wavelength, the photon will be absorbed by the atom, and the electron will jump from the lower energy level to the higher one. When the electron falls back down to its original state, the atom emits a photon of this same specific wavelength. So, only certain photons can be absorbed or emitted by an atom. The simplest atom is, of course, hydrogen with a single nuclear proton and a single electron.
Einstein's puzzled appearance in the image at the top of the page alludes to his discomfort with the rules of Quantum Mechanics, particularly the probabilistic nature of physics. Einstein is quoted as stating: "God does not roll dice!" But, to our best ability to determine, it seems clear that "She does!".

![]() Stellar Spectra
Stellar Spectra
![]() Space Telescopes
Space Telescopes
![]() Physics 7 Lectures
Physics 7 Lectures
![]() Physics 7 Home
Physics 7 Home
Conducted by Gene Smith, CASS/UCSD.
Comments?
You may send email to hsmith@ucsd.edu
Prof. H. E. (Gene) Smith
CASS 0424 UCSD
9500 Gilman Drive
La Jolla, CA 92093-0424
Last updated: 28 Jan 2000