The Units of Science
| H. E. Smith | Winter 2007 |

| Physics 7 The Units of Science |

Since Astronomy is one of the oldest sciences, it has developed many traditions over the years. While an astronomer would not hesitate for an instant to apply cutting-edge technology at the telescope or computer, the language and units of measurement in the field change much more slowly. So, as you set out to investigate the Universe with us, let us introduce you to a few words, units and short-hand that you'll encounter here.
 Scientific Notation
Scientific NotationAs was discussed in
The Scale
of the Universe tutorial, the important numbers in Astronomy span
almost 40 orders of magnitude in size. Consider
the mass of the Sun:
| It's cumbersome, to say the least, having to write out all of those zeros. Even kilograms (eliminate 3 zeros) or metric tons (eliminate 6 zeros) don't help much. Furthermore, we really don't know the Sun's mass beyond the accuracy of the fourth digit. All those zeros are just place-keepers, carrying no useful information. For this reason, scientists use a short-hand called Scientific Notation to express very large or very small numbers. |
 |
For very small numbers, such as the mass of the proton,
There are several good web pages about Scientific Notation. If you would like to read a bit more, try out the University of Maryland's Astronomy Programs site, with a Scientific Notation Exercise and an Astronomical Distance Calculator.
 Arithmetic in Scientific Notation
Arithmetic in Scientific NotationArithmetic with Scientific Notation is fairly straightforward, but requires treating the exponent separately. Suppose you wanted to estimate the mass of our Galaxy, the Milky Way. In round numbers there are about a half-trillion stars in the Milky Way:
 5 x 1011
5 x 1011

The Milky Way Galaxy - count the stars if you have a bit of free time.
so that an estimate of the mass of the Milky Way would be the number of
stars times the mass of a typical star:
 N*
x Msun
N*
x Msun  5 x 1011stars . 2 x 1033grams
5 x 1011stars . 2 x 1033grams
 ( 2 . 5 )
x 10 (11 + 33) grams
( 2 . 5 )
x 10 (11 + 33) grams  10
x 1044 grams = 1 x 1045grams
10
x 1044 grams = 1 x 1045grams
This is, of course, a crude estimate, especially because we now know that the mass of the Milky Way is dominated by unseen matter. Division in Scientific Notation is just the inverse process. Suppose you wanted to estimate the number hydrogen atoms is the sun. One estimate would be
 Msun / mH
Msun / mH  2 x 1033grams / 1.67 x 10-24grams
2 x 1033grams / 1.67 x 10-24grams
 2 x
10-24grams) and subtract the exponent of the divisor (-24) from
the exponent of the dividend (33).
2 x
10-24grams) and subtract the exponent of the divisor (-24) from
the exponent of the dividend (33).
 (2 / 2) x 10(33-[-24])
(2 / 2) x 10(33-[-24])  1
x 1057
1
x 1057
 Units of Measurement
Units of MeasurementBefore people can share information about the physical world, they need a common language and standard units of measurement. Since science is an international human endevour, scientists all over the world have agreed to use one set of units when they talk with each other about their work. This world-wide standard is called the metric system. So, when Scientist A in Katmandu says the distance to the Sun is 150,000,000 kilometers, Scientist B in Helsinki knows exactly what that means. Here is a list of fundamental units from the National Institute of Standards and Technology (NIST), formerly the National Bureau of Standards.
The metric system has standard prefixes to indicate relative sizes. As we noted above, Scientist A reported that the distance to the Sun was 150,000,000 kilometers. The prefix "kilo" means 1,000, so a kilo meter is 1,000 meters. The table below gives some metric names used in these pages and their definitions.
| Metric Prefixes | |||||
|---|---|---|---|---|---|
| PREFIX | DEFINITION | SCIENTIFIC NOTATION |
PREFIX | DEFINITION | SCIENTIFIC NOTATION |
| tera | 1,000,000,000,000 | 1012 | centi | .01 | 10-2 |
| giga | 1,000,000,000 | 109 | milli | .001 | 10-3 |
| mega | 1,000,000 | 106 | micro | .000001 | 10-6 |
| kilo | 1,000 | 103 | nano | .000000001 | 10-9 |
| deka | 10 | 101 | pico | .000000000001 | 10-12 |
| deci | .1 | 10-1 | femto | .000000000000001 | 10-15 |
Here is a comprehensive list of prefixes from the National Institute of Standards and Techonology (NIST).
A few examples of how to use the metric prefixes:
Even the "world standard" metric system has different versions. Astronomers use a version of the cgs (Centimeter-Gram-Second) system, modified for the immense distances in the Universe and huge masses of astronomical objects. Most physicists are converging on use of the MKS (Meter-Kilogram-Second) version or Systeme Internacionale.
 Length
Length
To describe distances and sizes, we define a standard of length.
The cgs unit of length is the centimeter, abbreviated as "cm". A
centimeter is between a third and a half an inch:
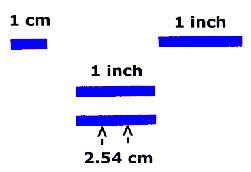
As you can see from the diagram, a centimeter is pretty small - not a
very practical unit for the enormous distances in the Universe. If our
Astronomer A had to use centimeters to tell Astronomer B the distance to the
Sun, it would look like this:
There are three special units of distance used by astronomers. These
are the astronomical unit (AU), the
light-year and the
parsec. The astronomical unit is the average distance of the Earth
from the Sun shown above.
A light-year (ly) sounds like a measure
of time, but it is a length - the distance light travels in one year.(We
can use a light-year as a unit of measure because ALL light travels at the
same speed; it is a fundamental constant of the Universe. More about this
later...) So, in one year, light travels:

The name parsec comes from the technique of measuring distance called
parallax. The nearest star, Alpha Centauri, is about 1.3 pc or
4 light-years away. We'll mostly stick to light-years in these pages, but
you may see stellar distances in pc, distances in the Milky Way Galaxy in
kiloparsecs (kpc), distances to other galaxies in megaparsecs (Mpc)
, and distances to very distant cosmological objects in gigaparsecs
(Gpc).
In addition to these distance units, astronomers use the
Ångstrom(Å) as a measure of size on the atomic scale.
 Mass
Mass
Everyone knows that the astronauts weigh less when they're walking on the Moon than when they're back on Earth. Since the Moon is less massive than the Earth, it's gravitational attraction is smaller. It is essential to have a unit for measuring "amount of stuff" that would be the same everywhere in the Universe. That unit of "stuff" is called mass. So, an astronaut's weight is less on the Moon, but his/her mass is exactly the same. Actually,weight and mass are two different things. Your weight is the gravitational attraction between you and the Earth. (Or whatever planet you may be visiting.) Your mass is a measure of your inertia, your resistance to changes in motion. Here is a nice explanation of mass.
The CGS unit of mass is the gram. It is
much smaller than an ounce:
On the very large (astronomical) scale and the very small (atomic) scale two other units of mass are used. To measure atomic masses the atomic mass unit (amu) is employed. the amu is defined as one-twelfth the mass of a common carbon atom:
slightly less than the mass of a proton. For stars, galaxies, etc. we use the solar-mass

 2 x 1033g
2 x 1033g
where the "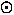 " is the standard astronomical
symbol for the sun. The mass of our Milky Way Galaxy is about
1012M
" is the standard astronomical
symbol for the sun. The mass of our Milky Way Galaxy is about
1012M .
.
 Time
Time
The CGS unit of time is the second. The
time standard is kept by the NIST using a cesium atomic clock like the
one to the right.
|
 Energy
Energy
Much of astronomy and astrophysics is concerned with understanding the energy generation and luminous energy output from stars, galaxies, etc. The cgs unit of energy is the erg. An erg is the amount of energy contained in the motion of a 1 gram mass moving with a speed of 1 centimeter/second or about the energy expended by a fly taking off from the wall.
The light output of a star or galaxy is called its luminosity measured in ergs/second. The luminosity of our sun
 = 4 x 1033 erg/s
= 4 x 1033 erg/s
At the atomic/nuclear level energies are sometimes quoted in electron-volts - eV, the energy of an electron accelerated through a voltage of 1 volt (1 eV = 1.6 x 10-12 erg). The energy-levels and ionization energies of common atoms are a few eV, x-ray photon energies are frequently quoted in kilo-electron-volts - keV, and gamma-rays and nuclear energies are frequently given in MeV. Because of the mass-energy equivalence implied by Einstein's
particle masses are sometimes quoted as the equivalent energy, usually in MeV. This is also called the "rest-energy" of the particle. The electron mass, me = 0.511 MeV and the proton mass, mp = 938 MeV. These are the energies that must be achieved by "atom-smashers" to create these particles out of pure energy.

![]() Through the Universe at the Speed of Light
Through the Universe at the Speed of Light
![]() Physics 7 Lectures
Physics 7 Lectures
![]() Physics 7 Home
Physics 7 Home